Hello, in this particular article you will provide several interesting pictures of fda grants eua for lilly's monoclonal antibody. We found many exciting and extraordinary fda grants eua for lilly's monoclonal antibody pictures that can be tips, input and information intended for you. In addition to be able to the fda grants eua for lilly's monoclonal antibody main picture, we also collect some other related images. Find typically the latest and best fda grants eua for lilly's monoclonal antibody images here that many of us get selected from plenty of other images.
 Covid, Fda grants authorization for Eli Lilly monoclonal antibodies We all hope you can get actually looking for concerning fda grants eua for lilly's monoclonal antibody here. There is usually a large selection involving interesting image ideas that will can provide information in order to you. You can get the pictures here regarding free and save these people to be used because reference material or employed as collection images with regard to personal use. Our imaginative team provides large dimensions images with high image resolution or HD.
Covid, Fda grants authorization for Eli Lilly monoclonal antibodies We all hope you can get actually looking for concerning fda grants eua for lilly's monoclonal antibody here. There is usually a large selection involving interesting image ideas that will can provide information in order to you. You can get the pictures here regarding free and save these people to be used because reference material or employed as collection images with regard to personal use. Our imaginative team provides large dimensions images with high image resolution or HD.
 Eli Lilly's Antibody Combo Wins FDA EUA for Mild to Moderate Covid-19 fda grants eua for lilly's monoclonal antibody - To discover the image more plainly in this article, you are able to click on the preferred image to look at the photo in its original sizing or in full. A person can also see the fda grants eua for lilly's monoclonal antibody image gallery that we all get prepared to locate the image you are interested in.
Eli Lilly's Antibody Combo Wins FDA EUA for Mild to Moderate Covid-19 fda grants eua for lilly's monoclonal antibody - To discover the image more plainly in this article, you are able to click on the preferred image to look at the photo in its original sizing or in full. A person can also see the fda grants eua for lilly's monoclonal antibody image gallery that we all get prepared to locate the image you are interested in.
 US FDA grants EUA to Eli Lilly's antibody therapies for Covid-19 We all provide many pictures associated with fda grants eua for lilly's monoclonal antibody because our site is targeted on articles or articles relevant to fda grants eua for lilly's monoclonal antibody. Please check out our latest article upon the side if a person don't get the fda grants eua for lilly's monoclonal antibody picture you are looking regarding. There are various keywords related in order to and relevant to fda grants eua for lilly's monoclonal antibody below that you can surf our main page or even homepage.
US FDA grants EUA to Eli Lilly's antibody therapies for Covid-19 We all provide many pictures associated with fda grants eua for lilly's monoclonal antibody because our site is targeted on articles or articles relevant to fda grants eua for lilly's monoclonal antibody. Please check out our latest article upon the side if a person don't get the fda grants eua for lilly's monoclonal antibody picture you are looking regarding. There are various keywords related in order to and relevant to fda grants eua for lilly's monoclonal antibody below that you can surf our main page or even homepage.
 FDA Expands EUA for Lilly's COVID-19 Monoclonal Antibody Hopefully you discover the image you happen to be looking for and all of us hope you want the fda grants eua for lilly's monoclonal antibody images which can be here, therefore that maybe they may be a great inspiration or ideas throughout the future.
FDA Expands EUA for Lilly's COVID-19 Monoclonal Antibody Hopefully you discover the image you happen to be looking for and all of us hope you want the fda grants eua for lilly's monoclonal antibody images which can be here, therefore that maybe they may be a great inspiration or ideas throughout the future.
 Lilly gets first EUA for monoclonal antibody treatment for children All fda grants eua for lilly's monoclonal antibody images that we provide in this article are usually sourced from the net, so if you get images with copyright concerns, please send your record on the contact webpage. Likewise with problematic or perhaps damaged image links or perhaps images that don't seem, then you could report this also. We certainly have provided a type for you to fill in.
Lilly gets first EUA for monoclonal antibody treatment for children All fda grants eua for lilly's monoclonal antibody images that we provide in this article are usually sourced from the net, so if you get images with copyright concerns, please send your record on the contact webpage. Likewise with problematic or perhaps damaged image links or perhaps images that don't seem, then you could report this also. We certainly have provided a type for you to fill in.
 FDA grants emergency authorization for Lilly antibody treatment - POLITICO The pictures related to be able to fda grants eua for lilly's monoclonal antibody in the following paragraphs, hopefully they will can be useful and will increase your knowledge. Appreciate you for making the effort to be able to visit our website and even read our articles. Cya ~.
FDA grants emergency authorization for Lilly antibody treatment - POLITICO The pictures related to be able to fda grants eua for lilly's monoclonal antibody in the following paragraphs, hopefully they will can be useful and will increase your knowledge. Appreciate you for making the effort to be able to visit our website and even read our articles. Cya ~.
 FDA Grants Lilly EUA for COVID-19 Antibody Bebtelovimab FDA Grants Lilly EUA for COVID-19 Antibody Bebtelovimab
FDA Grants Lilly EUA for COVID-19 Antibody Bebtelovimab FDA Grants Lilly EUA for COVID-19 Antibody Bebtelovimab
 FDA grants EUA to Lilly's Covid-19 antibody therapy for paediatric use FDA grants EUA to Lilly's Covid-19 antibody therapy for paediatric use
FDA grants EUA to Lilly's Covid-19 antibody therapy for paediatric use FDA grants EUA to Lilly's Covid-19 antibody therapy for paediatric use
 FDA Grants EUA to Eli Lilly's COVID-19 Antibody Therapy - YouTube FDA Grants EUA to Eli Lilly's COVID-19 Antibody Therapy - YouTube
FDA Grants EUA to Eli Lilly's COVID-19 Antibody Therapy - YouTube FDA Grants EUA to Eli Lilly's COVID-19 Antibody Therapy - YouTube
 FDA Authorizes Emergency Use of Lilly's COVID-19 Antibody Bamlanivimab FDA Authorizes Emergency Use of Lilly's COVID-19 Antibody Bamlanivimab
FDA Authorizes Emergency Use of Lilly's COVID-19 Antibody Bamlanivimab FDA Authorizes Emergency Use of Lilly's COVID-19 Antibody Bamlanivimab
 FDA Pulls Emergency Use Authorization for Regeneron and Eli Lilly FDA Pulls Emergency Use Authorization for Regeneron and Eli Lilly
FDA Pulls Emergency Use Authorization for Regeneron and Eli Lilly FDA Pulls Emergency Use Authorization for Regeneron and Eli Lilly

 FDA Issues EUA for Eli Lilly's Antibody Combination for Mild to FDA Issues EUA for Eli Lilly's Antibody Combination for Mild to
FDA Issues EUA for Eli Lilly's Antibody Combination for Mild to FDA Issues EUA for Eli Lilly's Antibody Combination for Mild to
 FDA authorizes Eli Lilly's monoclonal antibody treatment for COVID-19 FDA authorizes Eli Lilly's monoclonal antibody treatment for COVID-19
FDA authorizes Eli Lilly's monoclonal antibody treatment for COVID-19 FDA authorizes Eli Lilly's monoclonal antibody treatment for COVID-19
 Invivyd, FDA agree on steps to EUA for COVID monoclonal antibody Invivyd, FDA agree on steps to EUA for COVID monoclonal antibody
Invivyd, FDA agree on steps to EUA for COVID monoclonal antibody Invivyd, FDA agree on steps to EUA for COVID monoclonal antibody
 FDA grants EUA for COVID-19 monoclonal antibody treatment | Medical FDA grants EUA for COVID-19 monoclonal antibody treatment | Medical
FDA grants EUA for COVID-19 monoclonal antibody treatment | Medical FDA grants EUA for COVID-19 monoclonal antibody treatment | Medical
 Coronavirus treatment breakthrough? Eli Lilly's monoclonal antibody Coronavirus treatment breakthrough? Eli Lilly's monoclonal antibody
Coronavirus treatment breakthrough? Eli Lilly's monoclonal antibody Coronavirus treatment breakthrough? Eli Lilly's monoclonal antibody
 FDA revises Lilly's Covid-19 antibody combo EUA for use after exposure FDA revises Lilly's Covid-19 antibody combo EUA for use after exposure
FDA revises Lilly's Covid-19 antibody combo EUA for use after exposure FDA revises Lilly's Covid-19 antibody combo EUA for use after exposure
 FDA grants EUA to Regeneron antibody cocktail for Covid-19 FDA grants EUA to Regeneron antibody cocktail for Covid-19
FDA grants EUA to Regeneron antibody cocktail for Covid-19 FDA grants EUA to Regeneron antibody cocktail for Covid-19
 FDA approves emergency use of Eli Lilly COVID-19 monoclonal antibody FDA approves emergency use of Eli Lilly COVID-19 monoclonal antibody
FDA approves emergency use of Eli Lilly COVID-19 monoclonal antibody FDA approves emergency use of Eli Lilly COVID-19 monoclonal antibody
 FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment
FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment
 FDA Watch: Monoclonal Antibody Treatments for COVID-19, Preventive Flu FDA Watch: Monoclonal Antibody Treatments for COVID-19, Preventive Flu
FDA Watch: Monoclonal Antibody Treatments for COVID-19, Preventive Flu FDA Watch: Monoclonal Antibody Treatments for COVID-19, Preventive Flu
 BioNTech Unveils Modular mRNA Factories for Africa + FDA Authorizes Eli BioNTech Unveils Modular mRNA Factories for Africa + FDA Authorizes Eli
BioNTech Unveils Modular mRNA Factories for Africa + FDA Authorizes Eli BioNTech Unveils Modular mRNA Factories for Africa + FDA Authorizes Eli
 FDA grants Eli Lilly's coronavirus antibody drug emergency approval FDA grants Eli Lilly's coronavirus antibody drug emergency approval
FDA grants Eli Lilly's coronavirus antibody drug emergency approval FDA grants Eli Lilly's coronavirus antibody drug emergency approval
 Eli Lilly: Monoclonal antibodies benefit high-risk COVID-19 patients Eli Lilly: Monoclonal antibodies benefit high-risk COVID-19 patients
Eli Lilly: Monoclonal antibodies benefit high-risk COVID-19 patients Eli Lilly: Monoclonal antibodies benefit high-risk COVID-19 patients
 FDA revokes EUA for Lilly's Covid-19 antibody therapy bamlanivimab FDA revokes EUA for Lilly's Covid-19 antibody therapy bamlanivimab
FDA revokes EUA for Lilly's Covid-19 antibody therapy bamlanivimab FDA revokes EUA for Lilly's Covid-19 antibody therapy bamlanivimab
 FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment
FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment FDA grants EUA to Lilly's bebtelovimab for Covid-19 treatment
 US FDA on Twitter: "Today, we issued an EUA for new long-acting US FDA on Twitter: "Today, we issued an EUA for new long-acting
US FDA on Twitter: "Today, we issued an EUA for new long-acting US FDA on Twitter: "Today, we issued an EUA for new long-acting
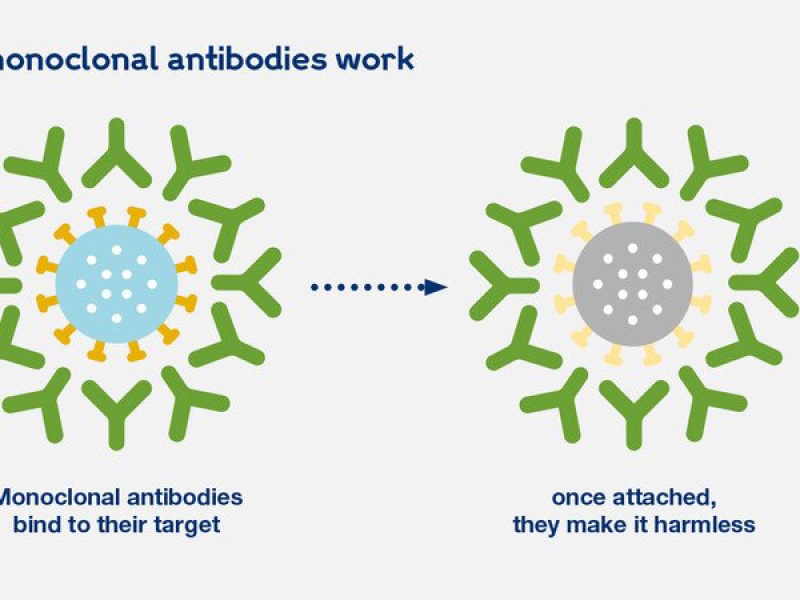 FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that
FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that
 Florida monoclonal antibody sites closed after FDA removes EUA | wtspcom Florida monoclonal antibody sites closed after FDA removes EUA | wtspcom
Florida monoclonal antibody sites closed after FDA removes EUA | wtspcom Florida monoclonal antibody sites closed after FDA removes EUA | wtspcom